Research
NanoFUSE Biologics are clinically proven to fuse the spine. The synergistic blend of osteoconductive, osteopromotive and anti-infective properties of bioactive ceramic 45S5 bioglass osteobiologic and osteoinductive property of DBM makes NanoFUSE clinically proven to be more effective than the competition and DBM alone
Featured Study
Int J Spine Surg . 2025 Dec 28;19(6):652-658.
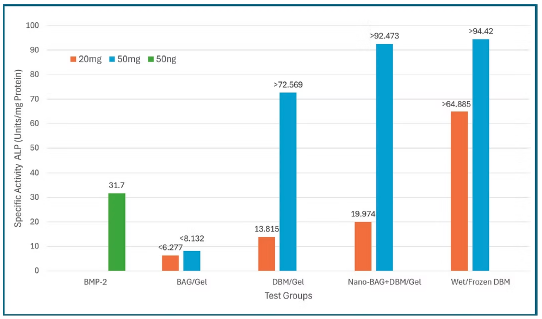
This in vitro study compared the osteoinductive potential of recombinant BMP-2, bioactive glass (BAG), demineralized bone matrix (DBM), and a composite nano-BAG + DBM formulation (NanoFuse DBM) using a C2C12 alkaline phosphatase assay. Results demonstrated that wet/frozen DBM produced the highest alkaline phosphatase activity, while NanoFuse DBM at higher concentration exceeded the assay’s detection limit and outperformed BMP-2, DBM + gel, and BAG-based formulations. BMP-2 showed comparatively lower osteogenic activity at the tested dose, and BAG alone exhibited minimal induction. Overall, the findings suggest that NanoFuse DBM provides robust, dose-dependent osteoinductive activity and may represent a safer and more effective alternative to BMP-2 and traditional graft materials for spinal fusion and other orthopedic applications, warranting further in vivo investigation.
Bioactive Glass Research
Proven to Heal Bone
Bioactive glass can act as a vehicle for delivering ions beneficial for healing and has been shown to regulate cells and improve bone growth and differentiation. Calcium released from the glass will react with phosphate ions present in the body fluids and deposit a nanocrystalline HA on the glass surface that mimics naturally formed HA by the process in which is made, the size, and morphology of the crystals.
Proven to Resorb Naturally
The bioactive glass in NanoFUSE is degradable in body fluids. The bioactive glass is chemically soluble, dissolving at rates ranging from ~2 to 5 μm per week.
Proven to be Anti-Infective
There is a lot of literature on the anti-infective properties of bioactive glass. There are several studies that show successful reduction of infection at the implant site. Bioactive glass is the only
anti-infective synthetic bone grafting material.
Proven to be Angiogenic
Trace ions in NanoFUSE bioactive glass composition such as copper and zinc are known to stimulate angiogenesis. Bioactive glasses containing these trace elements have been shown to stimulate vascular growth in vivo which can aid in the bone healing process.
Bibliography
F. Kirk, Gregg Ritter, Chad Waters, Sonoko Narisawa, Jose Luis Millan, James D. Talton. Osteoconductivity and Osteoinductivity of NanoFUSE
James F. Kirk, Gregg Ritter, Michael J. Larson, Robert C. Waters, Isaac finger, John Waters, John H. Abernethy, Dhyana Sankar, James D. Talton, and Ronald R. Cobb. Radiographic and Histologic Comparison of NanoFUSE® DBM and a Bioactive Glass in a Rabbit Spinal Fusion Model
Murrills RJ, Stein LS, Dempster DW. Stimulation of bone resoption and osteoclast clear zone formation by low pH: A time-course study. Cellular Physiology J 1993; vol154:3, 511-518.
Blue ML, The effects on cells because of changes in pH of body fluids. Seattlepi – online.
Arnett TR. Extracellular pH regulates bone cell function. Nutr J 2008, Feb; 138(2):415S-418S.
Galow AM, Rebl A, Koczan D, Bonk SM, Baumann W, Gisma J. Increased osteoblast viability at alkaline pH in vitro provides a new perspective on bone regeneration. Biochemistry and Biophysics Reports 2017, July; vol 10:17-25.
Hannink G, Chris Arts JJ. Bioresorbability, porosity, and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, Sept; vol 42:S22-S25.
LeGeros RZ, Lin S, Rohanizadeh R, Mijares D, LeGeros JP. Biphasic calcium phosphate bioceramics: preparation, properties, and applications. J Mater Sci Mater Med 2003, Mar;14(3)201-209.
Kyriazis V, Tzaphlidou M. Skeltal calcium/phosphorus ratio measuring techniques and results. I. Measuring and microtomography. Scientific World Journal 2004, 4, 1027-1034.
Yamada S, Heymann D, Bouler JM, Daculsi G. Osteoclastic resorptin of calcium phosphate ceramics with different hydroxyapatite/ β-tricalcium phosphate ratios. Biomaterials 1997, August; vol 18:1037-1041.
Friesenbichler J, Maurer-Ertl W, Sadoghi P, Pirker-Fruehauf U, Bodo K, Leithner A. Adverse reactions of artificial bone graft substitutes: Lessons learned from using tricalcium phosphate geneX®. Clin Orthop Relat Res 2014, Mar;472(3):976-982.
Favvas EP, Stefanopoulos KL, Vordos NC, Drosos GI, Mitropoulos AC. Structural characterization of calcium sulfate bone graft substitute cements. Mat Res 2016, Sept/Oct;vol 19 (5) online.
Kumar YC, Nalini KB, Menon J, Patro DP, Banerji BH. Calcium sulfate as bone graft substitute in the treatment of osseous bone defects, a prospective study. J Clin Diagn Res 2013, Dec;7(12):2926-2928.
Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, Tomsia AP. Bioactive glass in tissue engineering. Acta Biomat 2011, Mar;7:2355-2373.
Jung SB, Day DE. Conversion kinetics of silicate, borosilicate, and borate bioactive glasses to hydroxyapatite. Phys Chem Glass – Euro J Glass Sci and Tech 2009, April;vol 50(2):85-88.
Hench LL. The story of bioglass. J Mater Sci Mater Med 2006, Feb;17:967-978.
Malat TA, Glombitz M, Dahman J, Hax PM, Steinhausen E. The use of bioactive glass S53P4 as bone graft substitute in the treatment of chronic osteomylitis and infected non-unions – a retrospective study of 50 patients. Z Othrop Unfall 2008, April;156(2):152-159.
Roukema J, Rivron NC, Blitterswijik CA. Vascularization in tissue engineering. Trends Biotechnol 2008, Aug;26(8):434-441.
Fortier L, Bauer L, Chung E. Use of Fibergraft BG Morsels with BMA in anterior cervicval discectomy and fusion at 1, 2, 3, 4 levels: a retrospective analysis of fusion results. J Spine Neurosurg 2017, April;vol 6(3):1-3.
Fredericks D, Peterson EB, Watson N, Grosland N, Gibson-Corley K, Smucker J. Comparison of two synthetic bone graft products in a rabbit posterolateral fusion model. Iowa Orthrop J 2016, 36:167-173.
“In Vitro Human Umbilical Vein Endothelial Cells Response to Ionic Dissolution Products from Lithium-Containing 45S5 Bioactive Glass.” Haro Durand LA, Vargas GE, Vera-Mesones R, Baldi A, Zago MP, Fanovich MA, Boccaccini AR, Gorustovich A. Materials (Basel). 2017 Jul 3;10(7).
“Influence of 45S5 Bioactive Glass in A Standard Calcium Phosphate Collagen Bone Graft Substitute on the Posterolateral Fusion of Rabbit Spine.” Pugely AJ, Petersen EB, DeVries-Watson N, Fredericks DC. Iowa Orthop J. 2017;37:193-198.
“Development of a novel bioactive glass suitable for osteosarcoma-related bone grafts.” Yazdi, Alireza Rahimnejad, Lawrence Torkan, Stephen D. Waldman, and Mark R. Towler. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2017.
“A comparative in vivo evaluation of bioactive glasses and bioactive glass-based composites for bone tissue repair.” Bellucci, Devis, Alexandre Anesi, Roberta Salvatori, Luigi Chiarini, and Valeria Cannillo. Materials Science and Engineering: C79 (2017) Pages 286-295.
“Bioactive-glass in Oral and Maxillofacial Surgery,” A.C. Profeta and C. Huppa, Craniomaxillofac Trauma Reconstr. (2016) 9(1):1-14. Doi:10.1055.
“Biodegradable Mesoporous Bioactive Glass Nanospheres for Drug Delivery and Bone Tissue Regeneration,” Wang, X., & Li, W. (2016). Nanotechnology,27(22), 225102.
“Bioglass: 10 Milestones from Concept to Commerce,” Hench, L. L. (2016). Journal of Non-Crystalline Solids, 432, 2-8. doi:10.1016/j.jnoncrysol.2014.12.038
“Functionalized Mesoporous Bioactive Glass Scaffolds for Enhanced Bone Tissue Regeneration,” Deliang, Z., & Xinquan, J., Front. Bioeng. Biotechnol. Frontiers in Bioengineering and Biotechnology, (2016) doi:10.3389/conf.fbioe.2016.01.02648
“Injectable Bioative Glass in the Restoration of Oral Bone Defect,” C.B. Han, S.C An. Eur Rev Med Pharmacol Sci. (2016) 1665-8
“Investigation of Bioactivity, Biocompatibility and Thermal Behavior of Sol–gel Silica Glass Containing a High PEG Percentage,” Catauro, M., Renella, R., Papale, F., & Ciprioti, S. V., (2016) Materials Science and Engineering: C (2016) 61, 51-55.
“Mechanical Behaviour of Bioactive Glass Granules and Morselized Cancellous Bone Allograft in Load Bearing Defects,” Hulsen, D., Geurts, J., Gestel, N. V., Rietbergen, B. V., & Arts, J., Journal of Biomechanics, (2016). 49(7), 1121-1127.
“Nanoscale Bioactive Glass Activates Osteoclastic Differentiation of RAW 264.7 Cells,” Detsch, R., Rübner, M., Strissel, P. L., Mohn, D., Strasser, E., Stark, W. J., Boccaccini, A. R., Nanomedicine, 11(9) (2016) 1093-1105.
“Phosphate Glass Fibre Scaffolds: Tailoring of the Properties and Enhancement of the Bioactivity Through Mesoporous Glass Particles,” G. Novajra, N. G. Boetti, J. Lousteau, S. Fiorilli, D. Milanese and C. Vitale-Brovarone, Materials Science and Engineering, C (2016) 570-580.
“Three-Dimensional Polymer Coated 45S5-type Bioactive Glass Scaffolds Seeded With Human Mesenchymal Stem Cells Show Bone Formation in Vivo,” Westhauser, F., Weis, C., Prokscha, M., Bittrich, L. A., Li, W., Xiao, K., Moghaddam, A., J Mater Sci: Mater Med Journal of Materials Science: Materials in Medicine, 27(7) (2016) doi:10.1007/s10856-016-5732-3
“Enhanced Osteoprogenitor Elongated Collagen Fiber Matrix Formation by Bioactive Glass Ionic Silicon Dependent on Sp7 (Osterix) Transcription,” Varanasi, V. G., Odatsu, T., Bishop, T., Chang, J., Owyoung, J., & Loomer, P. M., Journal of Biomedical Materials Research Part A J. Biomed. Mater. Res.(2016).
“A Glass Fiber-Reinforced Composite – Bioactive Glass Cranioplasty Implant: A Case Study of an Early Development Stage Implant Removed Due to a Late Infection,” Posti, J. P., Piitulainen, J. M., Hupa, L., Fagerlund, S., Frantzén, J., Aitasalo, K. M., Vallittu, P. K. (2016) Journal of the Mechanical Behavior of Biomedical Materials, 55, 191-200.
“A Biomimetic Extracellular Matrix Composed of Mesoporous Bioactive Glass as a Bone Graft Material,” Hsu, F., Weng, R., Lin, H., Lin, Y., Lu, M., Yu, J., & Hsu, H. (2015). Microporous and Mesoporous Materials, 212, 56-65.
“Bioactive Borate Glass Promotes the Repair of Radius Segmental Bone Defects by Enhancing the Osteogenic Differentiation of BMSCs,” Zhang, J., Guan, J., Zhang, C., Wang, H., Huang, W., Guo, S., Wang, Y., Biomed. Mater. Biomedical Materials, 10(6), (2015) 065011.
“Bioactive Glass for Large Bone Repair,” W. Jia, G.Y. Lau, W. Huang, C. Zhang, A.P. Tomisa and Q. Fu, Adv Healthc Mater 4(18):2842-8 (2015).
“Bioactive Dlass for Long Bone Infection: A Systematic Review,” Aurégan, J., & Bégué, T, Injury, 46. (2015).
“Bioactive Glass in Cavitary Bone Defects: A Comparative Experimental Study in Rabbits,” Camargo, A. F., Baptista, A. M., Natalino, R., & Camargo, O. P. Acta Ortop. Bras. Acta Ortopédica Brasileira, 23(4), 202-207. (2015).
“Bioactive-glass in Periodontal Surgery and Implant Dentistry,” Profeta, A. C., & Prucher, G. M. Mater. J. Dental Materials Journal,34(5), 559-571. (2015).
“Bioactive Glasses Beyond Bone and Teeth: Emerging Applications in Contact with Soft Tissues,” Miguez-Pacheco, V., Hench, L. L., & Boccaccini, A. R. (2015). Acta Biomaterialia, 13, 1-15.
“Bioactive Glasses: Frontiers and Challenges,” Hench, L. L., & Jones, J. R.,Bioeng. Biotechnol. Frontiers in Bioengineering and Biotechnology,3. (2015)
“Bioactive Glasses – Structure and Properties,” D.S. Brauer, Angew Chem Int Ed Engl (2015). 54(14):4160-81.
“Biological Impact of Bioactive Glasses and Their Dissolution Products,” A. Hoppe and A.R. Boccaiccini, Front Oral Biol (2015).
“Characterization and Biocompatibility of a Fibrous Glassy Scaffold,” Gabbai-Armelin, P. R., Souza, M. T., Kido, H. W., Tim, C. R., Bossini, P. S., Fernandes, K. R., Renno, A. C. (2015). J Tissue Eng Regen Med Journal of Tissue Engineering and Regenerative Medicine.
“Effect of a New Bioactive Fibrous Glassy Scaffold on Bone Repair,” Gabbai-Armelin, P. R., Souza, M. T., Kido, H. W., Tim, C. R., Bossini, P. S., Magri, A. M., Renno, A. C. (2015). J Mater Sci: Mater Med Journal of Materials Science: Materials in Medicine, 26(5).
“Effect of Size of Bioactive Glass Nanoparticles on Mesenchymal Stem Cell Proliferation for Dental and Orthopedic Applications,” Ajita, J., Saravanan, S., & Selvamurugan, N. (2015). Materials Science and Engineering: C, 53, 142-149.
“Electrophoretic Deposition of Mesoporous Bioactive Glass on Glass–Ceramic Foam Scaffolds for Bone Tissue Engineering,” Fiorilli, S., Baino, F., Cauda, V., Crepaldi, M., Vitale-Brovarone, C., Demarchi, D., & Onida, B. (2015) J Mater Sci: Mater Med Journal of Materials Science: Materials in Medicine, 26(1).
“Emerging Developments in the Use of Bioactive Glass for Reconstruction of Craniofacial Bone,” Profeta, A. (2015). British Journal of Oral and Maxillofacial Surgery, 53(8), 760-762.
“Fiber Glass–Bioactive Glass Composite for Bone Replacing and Bone Anchoring Implants,” Vallittu, P. K., Närhi, T. O., & Hupa, L. (2015). Dental Materials, 31(4), 371-381.
“Improved Dimensional Stability with Bioactive Glass Fibre Skeleton in Poly(lactide-co-glycolide) Porous Scaffolds for Tissue Engineering,” Haaparanta, A., Uppstu, P., Hannula, M., Ellä, V., Rosling, A., & Kellomäki, M. (2015). Materials Science and Engineering: C, 56, 457-466.
“Silicon: The Evolution of its Use in Biomaterials,” Henstock, J., Canham, L., & Anderson, S. (2015). Acta Biomaterialia, 11, 17-26.
“The Future of Bioactive Ceramics,” Hench, L. L. (2015). J Mater Sci: Mater Med Journal of Materials Science: Materials in Medicine, 26(2).
“A Review of the Effect of Various Ions on the Properties and the Clinical Applications of Novel Bioactive Glasses in Medicine and Dentistry,” Ali, S., Farooq, I., & Iqbal, K. (2014). The Saudi Dental Journal, 26(1), 1-5.
“Bioactive Glasses with Improved Processing. Part 1. Thermal Properties, Ion Release and Apatite Formation,” Groh, D., Döhler, F., & Brauer, D. S. (2014).Acta Biomaterialia, 10(10), 4465-4473. doi:10.1016/j.actbio.2014.05.019
“Cotton-Wool-Like Bioactive Glasses for Bone Regeneration,” Poologasundarampillai, G., Wang, D., Li, S., Nakamura, J., Bradley, R., Lee, P., Jones, J. (2014). Acta Biomaterialia, 10(8), 3733-3746.
“Effect of Implant Design and Bioactive Glass Coating on Biomechanical Properties of Fiber-Reinforced Composite Implants,” Ballo, A. M., Akca, E., Ozen, T., Moritz, N., Lassila, L., Vallittu, P., & Närhi, T. (2014). Eur J Oral Sci European Journal of Oral Sciences, 122(4), 303-309.
“Evaluation of the Bone Regeneration Potential of Bioactive Glass in Implant Site Development Surgeries: A Systematic Review of the Literature,” Ioannou, A. L., Kotsakis, G. A., Kumar, T., Hinrichs, J. E., & Romanos, G. (2014). Clin Oral Invest Clinical Oral Investigations,19(2), 181-191.
“Healing of Critical-size Segmental Defects in Rat Femora Using Strong Porous Bioactive Glass Scaffolds,” Bi, L., Zobell, B., Liu, X., Rahaman, M. N., & Bonewald, L. F. (2014). Materials Science and Engineering: C,42, 816-824.
“Odontogenic Differentiation and Dentin Formation of Dental Pulp Cells Under Nanobioactive Glass Induction,” Wang, S., Gao, X., Gong, W., Zhang, Z., Chen, X., & Dong, Y. (2014). Acta Biomaterialia,10(6), 2792-2803.
“Review: Emerging Developments in the Use of Bioactive Glasses for Treating Infected Prosthetic Joints,” Rahaman, M. N., Bal, B. S., & Huang, W. (2014). Materials Science and Engineering: C, 41, 224-231.
“Review of bioactive glass: from Hench to hybrids”, Jones JR, Acta Biomater. 2013 Jan;9(1):4457-86. doi: 10.1016/j.actbio.2012.08.023. Epub 2012 Aug 21.
“A new synthesis route to high surface area sol gel bioactive glass through alcohol washing: a preliminary study”, Mukundan LM, Nirmal R, Vaikkath D, Nair PD. Biomatter. 2013 Apr-Jun;3(2). e24288-1 to e24288-10.
“Chronology of Bioactive Glass Development and Clinical Applications”, Larry L. Hench, New Journal of Glass and Ceramics, 2013, 3, 67-73
“Bioactive glass in tissue engineering”, Mohamed N. Rahaman, Delbert E. Day, B. Sonny Bal, Qiang Fu, Steven B. Jung, Lynda F. Bonewald, and Antoni P. Tomsia, Acta Biomater. 2011 June ; 7(6): 2355–2373.
“Development and Applications of Varieties of Bioactive Glass Compositions in Dental Surgery, Third Generation Tissue Engineering, Orthopaedic Surgery and as Drug Delivery System”, Samit Kumar Nandi, Biswanath Kundu and Someswar Datta, Chapter 4, Biomaterials Applications for Nanomedicine. Edited by Rosario Pignatello, ISBN 978-953-307-661-4, 470 pages, Publisher: InTech, Chapters published November 16, 2011 under CC BY 3.0 license.
“Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering”, Lutz-Christian Gerhardt and Aldo R. Boccaccini, Materials 2010, 3, 3867-3910.
“Enhanced osteocalcin expression by osteoblast-like cells (MC3T3-E1) exposed to bioactive coating glass (SiO2-CaO-P2O5-MgO-K2O-Na2O system) ions”, Varanasi VG1, Saiz E, Loomer PM, Ancheta B, Uritani N, Ho SP, Tomsia AP, Marshall SJ, Marshall GW, Acta Biomater. 2009 Nov;5(9):3536-47.
“Genetic design of bioactive glass” Larry L. Hench, Journal of the European Ceramic Society, 29 (2009) 1257–1265.
“Bioactive glass as a bone substitute for spinal fusion in adolescent idiopathic scoliosis; a comparative study with iliac crest autograph”, Ilharreborde B, Morel E, Fitoussi F, et al. J Pediatr Orthop 2008:28;347-351.
“Growth and differentiation of osteoblastic cells on 13-93 bioactive glass fibers and scaffolds” Brown RF1, Day DE, Day TE, Jung S, Rahaman MN, Fu Q, Acta Biomater. 2008 Mar;4(2):387-96.
“Antimicrobial effect of nanometric bioactive glass 45S5”, Waltimo T, Brunner TJ, Vollenweider M, Stark WJ, Zehnder M. J Dent Res. 2007 Aug;86(8):754-7.
“Bioactive Glass Scaffolds for Bone Regeneration”, Julian R. Jones, Eileen Gentleman and Julia Polak, Elements, Vol. 3, PP 393-399, December 2007.
“Optimising bioactive glass scaffolds for bone tissue engineering”, Julian R. Jones, Lisa M. Ehrenfried, Larry L. Hench, Biomaterials 27 (2006) 964–973
“Bioglass®: A short history and bibliography”, L. L. Hench, June W. Hench and D. C. Greenspan, J. Aust. Ceram. Soc., [40], 1, 2004, pages 1-42.
“Bioactive evaluation of 45S5 bioactive glass fibers and preliminary study of human osteoblast attachment”, Clupper DC, Gough JE, Embanga PM, Notingher I, Hench LL, Hall MM. J Mater Sci Mater Med. 2004 Jul;15(7):803-8.
“Osteoblast attachment and mineralized nodule formation on rough and smooth 45S5 bioactive glass monoliths”, Gough JE, Notingher I, Hench LL, J Biomed Mater Res A. 2004 Mar 15;68(4):640-50.
“Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: results of a 12-month clinical and radiological study”, R Mengel, M Soffner, L Flores-de-Jacoby, J Periodontol, 74;6:899-908, June (2003).
“Indirect cytotoxicity evaluation of soluble silica, calcium and phosphate ions”, J. Selvakumaran, P.Saravanapavan and L.L. Hench, Bioceramics 16International Symposium on Ceramics in Medicine (2003).
“Bioactive Materials for Tissue Engineering Scaffolds”, Larry L Hench, Julian R Jones, Pilar Sepulveda, Chapter 1. Future Strategies for Tissue and Organ Replacement, Edited by: Julia M Polak, Larry L Hench, P Kemp , 448pp, May 2002. ISBN: 978-1-86094-964-7.
“Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution”, Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM, J Biomed Mater Res. 2001 May;55(2):151-7.
“Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: implications and applications for bone tissue engineering”, Xynos ID, Hukkanen MV, Batten JJ, Buttery LD, Hench LL, Polak JM, Calcif Tissue Int. 2000 Oct;67(4):321-9.
“A bioactive glass particulate in the treatment of molar furcation invasion”, CR Anderegg, DC Alexander, M Freidman, (1999)J Periodontol, 70; 4:384-7.
“Histologic Observations of Periodontal Wound Healing After Treatment with PerioGlasÔ in Non-Human Primates,” S. Karatzas, A. Zavras, D. Greenspan, S. Amar, (1999) Intl J Perio Res Den, 19 (5).
“Clinical evaluation of bioactive glass in the treatment of periodontal osseous defects in humans”, TB Lovelace, JT Mellonig, RM Meffert, AA Jones, PV Nummikoski, DL Cochran, J Periodontol, (1998) 69; 9:1027-35.
“Comparison of BioglassÒ Synthetic Bone Graft Particles and Open Debridement in the Treatment of Human Periodontal Defects”, J. Froum , S.C. Cho, E. Rosenberg, M. Roher, and D. Tarnow, J. Perio; (1998) 69:698-709.
“An evaluation of bioactive ceramic in the treatment of periodontal osseous defects”, SB Low, CJ King, J Krieger, Int J Periodontics Restorative Dent, 17;4:358-67, August (1997).
“Analysis of the chemical transformation of bioactive glass particles after implantation”, E Schepers, L Barbier, A Huygh, P Ducheyne, J Oral Rehabilitation, 24: 171-181, (1997).
“Behavior of Bioactive Glass (S53P4) in Human Frontal Sinus Obliteration”, K Aitasalo, J Suonpaa, M Peltola, A Yli-Urpo, Bioceramics, 10: 429-32, October (1997).
“Clinical use of a bioactive glass particulate in the treatment of human osseous defects”, CA Shapoff, DC Alexander, AE Clark, Compend Contin Educ Dent, 18;4:352-4, April (1997).
“Particulate Bioglass® Compared with Hydroxyapatite as a Bone Graft Substitute,” H. Oonishi, S. Kutrshitani, E. Yasukawa, H. Iwaki, L. L. Hench, J. Wilson, E. Tsuji, and T. Sugihara, Clinical Orthopaedics and Related Research, 334 (1997), 316-325.
“Using 45S5 Bioglass® Cones as Endosseous Ridge Maintenance Implants to Prevent Alveolar Ridge Resorption: A 5-Year Evaluation,” Harold R. Stanley, Matthew B. Hall, Arthur E. Clark, Caleb J. King III, Larry L. Hench, Joseph J. Berte, J. Oral Maxillofac. Implants, 12 (1997), 95-105.
“Ossicular Replacement Prostheses”, K. Lobel, in Clinical Performance of Skeletal Prostheses, Hench and Wislon (eds), Chapman and Hall, Ltd, London, (1996), pp. 214-236.
“Alveolar Ridge Maintenance Implants” H.R.Stanley, A.E.Clark, June Wilson and L.L.Hench. Clinical Performance of Skeletal Prostheses. L.L.Hench and June Wilson, Eds. Chapman and Hall, Publishers,London, England (1995).
“Bioglass® Middle Ear Devices: Ten year Clinical Results” June Wilson, Ellis Douek and Kevin Rust. Bioceramics 8, (1995) June Wilson, L.L.Hench and D. Greenspan, Eds. Elsevier Science, Oxford, England, Publishers.
“Bone Growth into Spaces Between 45S5 Bioglass® Granules,” H. Oonishi, S. Kushitani, E. Yasukawa, H. Kawakami, A. Nakata, S. Koh, L. L. Hench, J. Wilson, E. Tsuji, and T. Sugihara, in Bioceramics 7, Ö. H. Andersson and A. Yli-Urpo, eds., Butterworth-Heinemann Ltd., Oxford, England (1994), 139-144.
“Periodontal Repair Using Perioglas® in NonHuman Primates: Clinical and Histologic Observations”, A.E. Fetner, M.S. Hartigan, S.B.Low, Compendium Contin Educ Dent, 15(7), 932-939, 1994.
“Tissue Response to Bioglass® Endosseous Ridge Maintenance Implants,” June Wilson, A. E. Clark, M. Hall and L. L. Hench, Oral Implantology, XIX[4], (1993), 295-302.
“Bioactive Ceramics for Periodontal Treatment: Comparative Studies.” June Wilson and S.B.Low. Applied Biomaterials, Vol.3, (1992) 123-129.
“Bioactive glass particulate material as a filler for bone lesions”, E Schepers, M de Clercq, P Ducheyne, Journal of Oral Rehabilitation, 18: 439-452, (1991).
“Otologic Applications of Bioglass® Implants,” E. Douek, in Proceedings of IVth International Symposium on Bioceramics in Medicine. Edited by W. Bonfield, London, Sept. 10-11, (1991).
“Bonding of Soft Tissues to Bioglass®”, J. Wilson and D. Noletti in Handbook of Bioactive Ceramics Vol. 1: bioactive Galsses and Glass-Ceramics,Yamamuro, L.L. Hench and J. Wilson, eds., CRC Press, Boca Raton, FL (1990).
“Review of Bioactive Materials for Otologic and Maxillofacial Applications”; G. E. Merwin, in Handbook of Bioactive Ceramics, Vol I. Edited by T. Yamamuro, L. L. Hench and J. Wilson. CRC Press, Boca Raton, FL, (1990), pp. 323-328.
“Bioactive Materials for Periodontal Treatment: A Comparative Study,” J. Wilson, S. Low, A. Fetner and L. L. Hench in Biomaterials and Clinical Applications, Pizzoferrato, P. G. Marchetti, A. Ravaglioli and A.J.C. Lee, eds., Elsevier Science Publishers, Amsterdam, (1987), 223-228.
“Early Clinical Trials of 45S5 Bioglass® for Endosseous Ridge Maintenance with a New Endosseous Implant Material”, M.B. Hall, H.R. Stanley, C.King, F. Colaizzi, D. Spilman and L.L. Hench, Prosthetic Dentistry, 58 [5] (1987), 607-613.
“Residual Alveolar Ridge Maintenance With a New Endosseous Implant Material”, H.R. Stanley, M.B. Hall, F. Colaizzi and A.E. Clark, Jr. in Maxillofacial Prosthetics, Dental Implants, Adisman, and R.P. Desjardins, eds. J. Prosthetic Dentistry 58 [5] (1987).
“Bioglass® middle ear prosthesis: preliminary report”, GE Merwin, Ann Otol Rhinol Laryngol, 95; 1 Pt 1:78-82 January-February (1986).
“Facial Bone Augmentation Using Bioglass® in Dogs”, J. Wilson, G. Merwin,L. Rogers, D.Spilman and R. Martin, in Surgical Research Recent Development, Proc. 1st Annual Scientific Session of Acad. Of Surgical Res., W. Hall, ed. Pergamon Press, (1985), 66-70
Histopathological Evaluation of Interaction between Tympanic Membrane and Implant Materials”, June Wilson, J.S.Atkins, G.E.Merwin and L.L.Hench.Soc. For Biomat. Vol.8 195, 1985
“Tissue Response to Surface Active Material”, J. Wilson and L. L. Hench, in The Dental Implant, R.V. McKinney Jr and J.E. Lemons eds., PSG Publishing Co. (1985) 181-196.
“Bioglass Implants for Otology,” L. L. Hench, June Wilson and G. Merwin in Biomaterials in Otology, J. Grote, ed., Martinus Nijhoff Publishers, The Hague-Boston-London, 1983.
“Comparison of Ossicular Replacement Materials in a Mouse Ear Model,” Gerald E. Merwin, James S. Atkins, June Wilson, Larry L. Hench, Head Neck Surg., 90 (1982) 461-469.
“The Implantation of Natural Tooth Form Bioglass® in Baboons – Long Term Results,” H. R. Stanley, L. L. Hench, C. G. Bennett, Jr., S. J. Chellemi, C. J. King, III, R. E. Going, N. J. Ingersoll, E. C. Ethridge, K. L. Kreutziger, L. Loeb, and A. E. Clark, Intern. Oral Implantology 2 (1981) 26-36.
“Toxicology and Biocompatibility of Bioglasses®,” J. Wilson, G. H. Pigott, F. J. Schoen and L. L. Hench, Biomed. Maters. Res. 15 (1981) 805-817.
“Implantation of Natural Tooth Form Bioglasses in Baboon”, H.R. Stanley et.al., J. Oral Implantology, 1:2 (1976)
“The Implantation of Natural Tooth Form Bioglasses in Baboons,” H. R. Stanley, L. L. Hench, R. Going, C. Bennett, S. J. Chellemi, C. King, N. Ingersoll, E. Ethridge, and K. Kreutziger, Oral Surg., Oral Med., Oral Pathology 45[5] (1976) 339-356
“Histo-Chemical Responses at a Biomaterials Interface,” L. L. Hench and H. A. Paschall, Biomed. Mats. Res., No. 5 (Part 1) (1974) 49-64.
“Direct Chemical Bonding of Bioactive Glass-Ceramic Materials and Bone,” L. L. Hench and H. A. Paschall, Biomed. Mats. Res. Symp. No. 4 (1973) 25-42.